
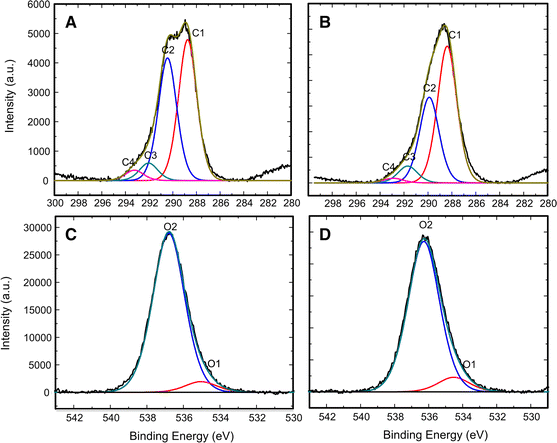
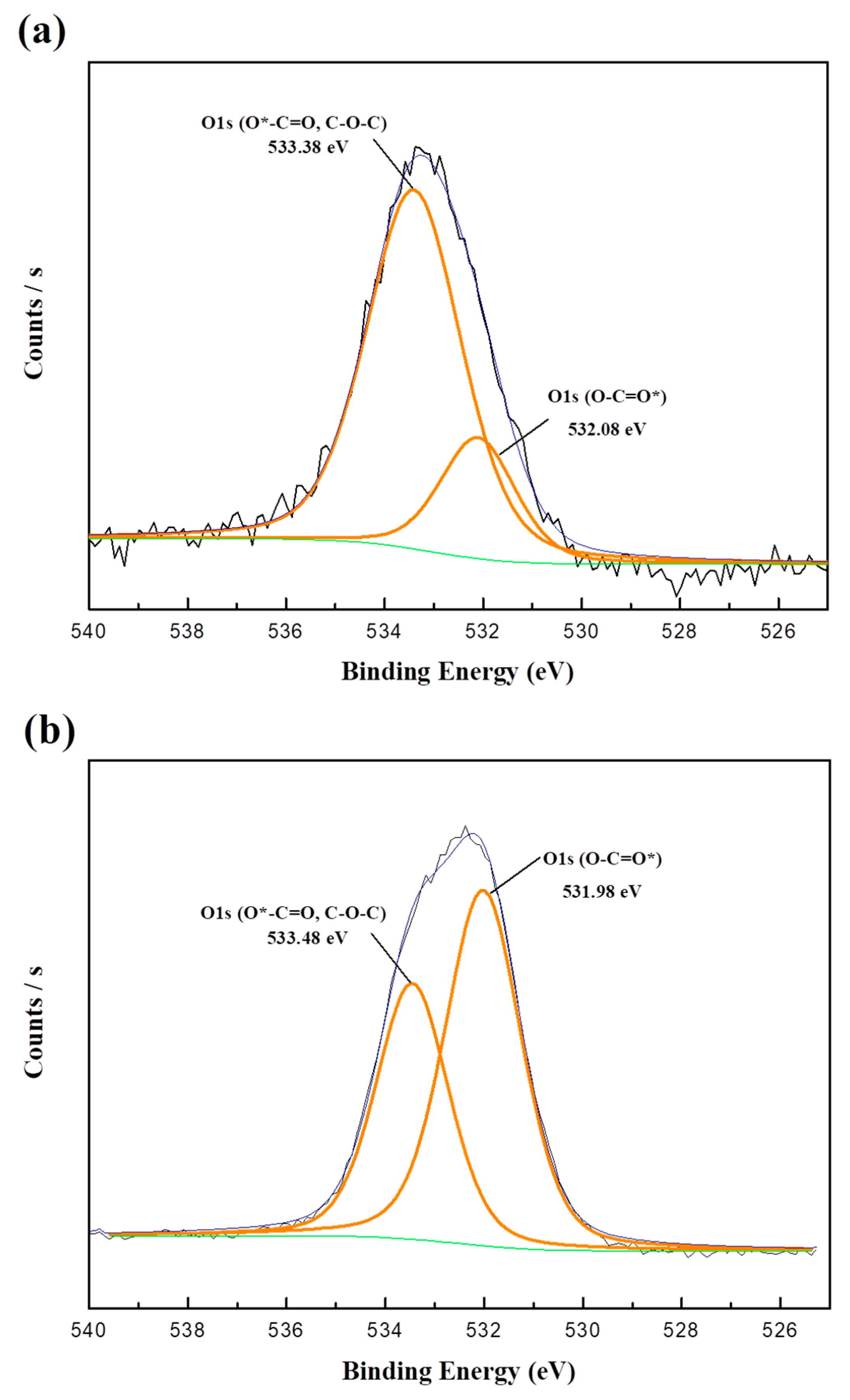
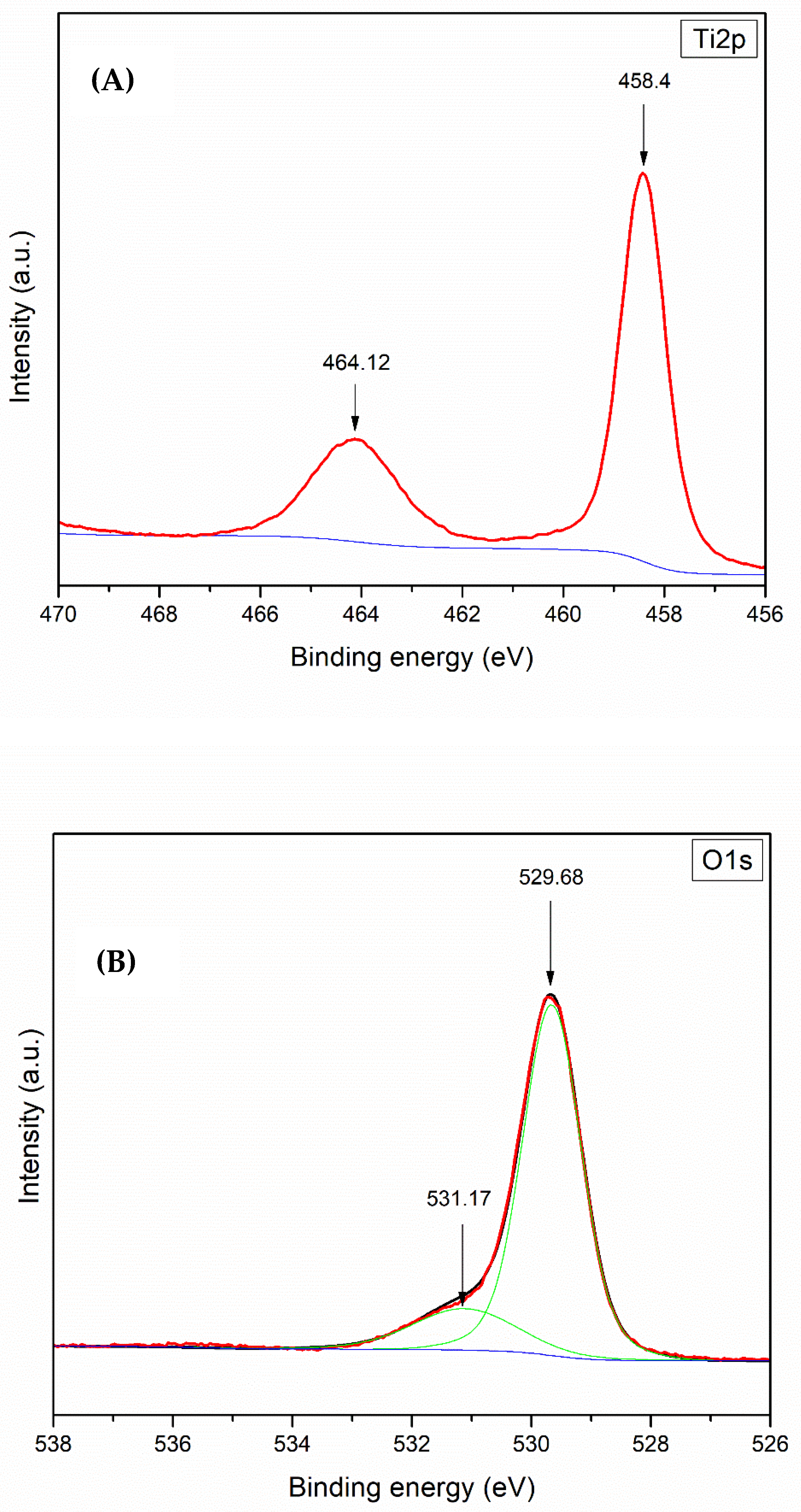
The OA-VA processes exhibit the relation of residual O i and additional V o. SA-ZNRs annealed in VA-OA processes reveal positive correlations between the oxygen flow rate and formation of oxygen interstitials (O i) and zinc vacancies (V Zn). We demonstrate the strategic two step annealing process to create reliable structural configuration in SA-ZNRs during the first round of annealing at 800 ☌ in vacuum (VA process), and create intrinsic defects in the second step of annealing in oxygen rich atmosphere (OA process) to correlate the formation of the defects related to green/orange-red emission. Moulder (1992) shows examples of these standards to help interpret the spectra.Although, post annealing is an efficient way to annihilate/restructure deficiencies in self-assembly (SA) ZnO nanorods (ZNRs), the detailed investigation about the surface properties of annealed SA-ZNRs is a long standing issue and the major discrepancy is mainly due to single step annealing. Some instruments have peak identification features, but otherwise, the identification of peaks/lines on the spectra can be completed by looking at standards of different materials. Electrons in 2s will have greater energy than those in 2p. peaks from electrons in 1s will have a greater energy than peaks representing electrons from 2s. The greater the binding energy, the greater the attraction of that electron to the nucleus. As seen in the figure above, the O1s has the largest peak, and it shows that the atomic composition of oxygen is the greatest. Therefore, the peak intensities give information about the percent composition of a material.
For example, if a peak, A, is half the height of another peak B, that means there were half as many electrons detected with the binding energy at A compared to the number of electrons detected with the binding energy at B. The shorter the peak, the less electrons represented. Peaks from the XPS spectra give relative number of electrons with a specific binding energy. (Figure courtesy of the creative commons license. Graph showing the binding energies of electrons from different orbitals (F1s, O1s, Si2p, etc.) and their intensities which tell the atomic composition of the sample based on the amounts of each electron from different orbitals present. Electrons of different energies follow different paths through the detector which allows the computer to differentiate the electrons and produce the spectra seen below.įigure \(\PageIndex\): XPS spectra. The energy of those ejected electrons is analyzed by a detector and a plot of these energies and relative numbers of electrons is produced.
X-rays (photons) are shot onto a sample, and when electrons in the sample absorb enough energy, they are ejected from the sample with a certain kinetic energy.


 0 kommentar(er)
0 kommentar(er)
